
PEOPLE in the North-East are split on whether to get vaccinated against Covid, with questions raised around safety, cost and availability.
The vaccine from pharmaceutical giant Pfizer, working with German biotech company BioNTech, has been approved for use in the UK - meaning thousands in the North-East and across the country could be vaccinated.
But that's not the only vaccine the UK has bought.
Here's everything you need to know about Pfizer vaccine plus key questions like who's first in line to be vaccinated and how it will be rolled out.
Is this a reason to celebrate?
Yes. Analysis shows the vaccine can prevent 95 per cent of people from getting Covid-19, including 94 per cent in older age groups.
The vaccine has been tested on 43,500 people in six countries and no safety concerns were raised.
Approval means the UK can begin rolling out the vaccine to those most in need, including frontline NHS workers.

What type of vaccine is this?
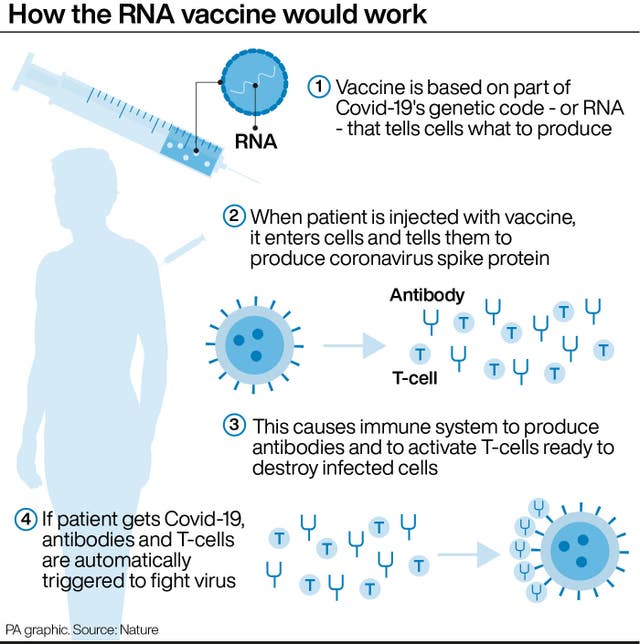
The jab is known as a messenger RNA (mRNA) vaccine.
Conventional vaccines are produced using weakened forms of the virus, but mRNAs use only the virus’s genetic code.
An mRNA vaccine is injected into the body where it enters cells and tells them to create antigens.
These antigens are recognised by the immune system and prepare it to fight coronavirus.
What are the advantages of this type of vaccine?
No actual virus is needed to create an mRNA vaccine. This means the rate at which it can be produced is dramatically accelerated.
 (PA Graphics)
(PA Graphics)
As a result, mRNA vaccines have been hailed as potentially offering a rapid solution to new outbreaks of infectious diseases.
In theory, they can also be modified reasonably quickly if, for example, a virus develops mutations and begins to change.
mRNA vaccines are also cheaper to produce than traditional vaccines, although both will play an important role in tackling Covid-19.
One downside to mRNA vaccines is that they need to be stored at ultra-cold temperatures and cannot be transported easily.
Are they safe?
All vaccines undergo rigorous testing and have oversight from experienced regulators.
UPDATE: We are proud to announce, along with @BioNTech_Group, that our mRNA-based #vaccine candidate has, at an interim analysis, demonstrated initial evidence of efficacy against #COVID19 in participants without prior evidence of SARS-CoV-2 infection.
— Pfizer Inc. (@pfizer) November 9, 2020
Some believe mRNA vaccines are safer for the patient as they do not rely on any element of the virus being injected into the body.
mRNA vaccines have been tried and tested in the lab and on animals before moving to human studies.
The human trials of mRNA vaccines – involving tens of thousands of people worldwide – have been going on since early 2020 to show whether they are safe and effective.
Pfizer will continue to collect safety and long-term outcomes data from participants for two years.
Do we have enough doses to vaccinate the UK population?
The UK has secured 40 million doses of the Pfizer/BioNTech vaccine, with 10 million due in the UK by the end of the year.
Patients need two doses, meaning not enough shots have been secured for the entire UK population.
However, it is likely other vaccines, including one from Oxford University, will be approved in the coming weeks and months.
The UK has secured access to:
- 60 million doses of the Novavax vaccine
- Some 30 million doses from Janssen
- 40 million doses of the Pfizer/BioNTech vaccine – the first agreement the firms signed with any government
- Sixty million doses of a vaccine being developed by Valneva
- 60 million doses of protein adjuvant vaccine from GlaxoSmithKline (GSK) and Sanofi Pasteur
- Five million doses of the jab on offer from Moderna in the US
How will a vaccine be rolled out?
Work has been going on behind the scenes to ensure that NHS staff are ready to start delivering jabs to the most vulnerable, as well as health and care workers, as a priority.
The NHS Nightingale Hospitals have also been earmarked as sites for mass vaccination clinics – among other uses.
In addition, NHS leaders have said there will be “roving teams” deployed to vaccinate care home residents and workers.
Based on the current information, the vaccines being developed require two doses per patient, with a 21-day gap between doses.
New regulations allowing more healthcare workers to administer flu and potential Covid-19 vaccines have also been introduced by the Government.

Who is top of the list to get a coronavirus vaccine?
The Joint Committee on Vaccination and Immunisation (JCVI) has examined data on who suffers the worst outcomes from coronavirus and who is at highest risk of death.
But who will get a jab could depend on how easily one can be rolled out, with the Pfizer jab needing storage temperatures of minus 70C to minus 80C.
For now, the JCVI’s interim guidance says the order of priority should be:
- Older adults in a care home and care home workers
- All those who are 80 years of age and over and health and social care workers
- All those who are 75 years of age and over
- All those who are 70 years of age and over and clinically extremely vulnerable individuals, excluding pregnant women and those under 18 years of age
- All those who are 65 years of age and over
- Adults aged 18 to 65 years in an at-risk group
- All those aged 50 and over
Don’t vaccines take a long time to produce?
In the past it has taken years, sometimes decades, to produce a vaccine.
Traditionally, vaccine development includes various processes, including design and development stages followed by clinical trials – which in themselves need approval before they even begin.
But in the trials for a Covid-19 vaccine, things look slightly different. A process which usually takes years has been condensed to months.
While the early design and development stages look similar, the clinical trial phases overlap, instead of taking place sequentially.
And pharmaceutical firms have begun manufacturing before final approval has been granted – taking on the risk that they may be forced to scrap their work.
The new way of working means that regulators around the world can start to look at scientific data earlier than they traditionally would do.

What do they cost?
Pfizer/BioNTech is making its vaccine available not-for-profit.
According to reports, the Moderna vaccine could cost about 38 dollars (£28) per dose and the Pfizer candidate could cost around 20 dollars (£15).
Researchers suggest the Oxford vaccine could be relatively cheap to produce, with some reports indicating it could be about £3 per dose.
AstraZeneca said it will not sell it for a profit, so it can be available to all countries.
However, the details of the deals made by the UK Government have not been made public.



Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel