
The coronavirus vaccine from Pfizer – which is due to arrive in the UK before the end of the year – is 95 per cent effective and has passed its safety checks, according to further data from the firm.
The pharmaceutical giant and its partner BioNTech published interim results last week showing the jab could prevent more than 90% of people developing Covid-19.
That data was based on the first 94 volunteers to develop Covid-19, but further figures released on Wednesday are based on the first 170 cases of the virus in the clinical trial.
- Covid: Ministers must 'come clean' over failings, says watchdog
- Tees Valley mayor welcomes green agenda amid ban on new petrol and diesel vehicle sales by 2030
- Covid: Northern Echo readers torn over whether to get a vaccine
Of these 170 Covid-19 cases, 162 were observed in the placebo group versus eight in the vaccine group.
A good immune response was “consistent across age, gender, race and ethnicity demographics” and the jab was over 94 per cent effective in those aged over 65, Pfizer said.
Of those taking part in the trial, 42 per cent were from diverse ethnic backgrounds and 41 per cent were aged between 56 and 85.
There were 10 severe cases of Covid-19 overall, with nine in the placebo group and one in the vaccine group.
The vaccine has been tested on 43,500 people in six countries and no safety concerns have been raised, although around 2 per cent of those involved suffered a headache and fatigue.
The UK has secured 40 million doses in total of the vaccine, with 10 million due in the country by the end of the year if the jab is approved.
People will need two doses, meaning not enough vaccine has been secured for the entire UK population.
Another jab, from US firm Moderna, was shown this week in early data to be almost 95 per cent effective.
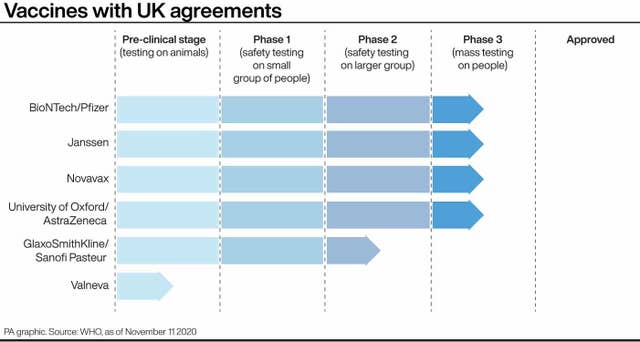
The UK has ordered five million doses of that jab, and also awaits the results of the Oxford University and AstraZeneca vaccine study – which is due to report soon.
Changes to the Human Medicine Regulations announced in October will allow the Medicines and Healthcare products Regulatory Agency (MHRA) to authorise temporary supply of vaccines, if one becomes available before 2021.
This means that if a vaccine is found to meet the safety, quality and effectiveness standards by the MHRA then vaccinations can begin without needing to wait for the European Medicines Agency.
Health Secretary Matt Hancock has said the NHS will be ready by December 1 to roll out any jab.
 (PA Graphics)
(PA Graphics)
The Pfizer vaccine has been shown to produce both an antibody and T-cell response in the body to fight coronavirus.
Pfizer and BioNTech expect to be able to produce up to 50 million vaccine doses globally in 2020 and up to 1.3 billion in 2021.
Pfizer chief executive Albert Bourla said on Tuesday that the firm is preparing to file for emergency use authorisation within days from the US Food and Drug Administration.
On Wednesday, Mr Bourla said: “The study results mark an important step in this historic eight-month journey to bring forward a vaccine capable of helping to end this devastating pandemic.
“We continue to move at the speed of science to compile all the data collected thus far and share with regulators around the world.
“With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, co-founder of BioNTech, said the data shows a high rate of protection against Covid-19 can be achieved “very fast” after the first dose.



Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel